Cleaning detergents are made up of surfactants as the main ingredient. Surfactants are a term that refers to surface active agent. Surfactants are active agents that trigger surface activity and improve dirt removal and trapping of dirt from surfaces.
Surfactants come with a hydrophobic tail (water-hating) and a hydrophilic head (water-loving). The hydrophobic tails of all surfactants surround soils. The hydrophilic head is surrounded by water.
What is the basic principle of surfactants?
When there are enough surfactant molecules present in a solution they combine together to form micelles. The micelle formation is initiated by the surfactant heads, which position themselves so that they are exposed to water. While the tails remain secure within the center of the structure, they are kept out from water. When you desire a knockout post on nonionic surfactant suppliers, browse around this website.
Micelles can be used as a unit for removing soils. The hydrophobic tails draw attention to soils, and they surround them while the hydrophilic heads lift the surrounded soils off the surface and into the cleaning solution. The micelles then form with the tails suspended by the soil at the center of the structure.
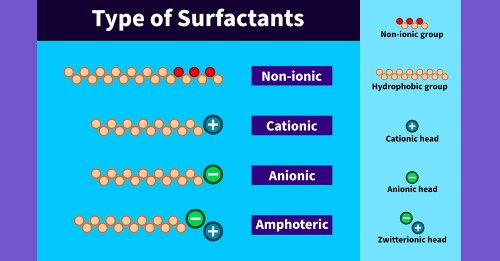
Types of Surfactants
The hydrophilic head of each surfactant is charged electrically. The charge may be negative, positive, or neutral. The charge depends on the head that is hydrophilic and the type of surfactant, it is classified as anionic, nonionic, amphoteric or cationic.
Anionic Surfactants
Anionic surfactants carry negative charges on their hydrophilic end. Surfactant molecules have negative charges on their hydrophilic ends. This assists in lifting and hold soils in micelles. Because they are able to attack a broad range of soils anionic surfactants are employed frequently in soaps and detergents. IRO Surfactant create a lot of foam when mixed. Anionic surfactants can lift and suspend soil particles, but they are not good in emulsifying oily soils.
Nonionic Surfactants
Nonionic surfactants have a neutral pH and no hydrophilic charge. Nonionic surfactants are very good at emulsifying oils and are better than anionic surfactants in removing organic soils. They are often combined to make dual-action, multi-purpose cleaners which can not just lift and suspend particulate soils as well as make oily soils more emulsifiable.
Nonionic surfactants can be non-foaming, or low-foaming. They're a good option for detergents with low foaming because of this.
Nonionic surfactants have a unique characteristic that is known as a cloud point. The temperature at which nonionic surfactants begin to separate from cleaning solutions is called the cloud point. When this occurs, the cleaning solution will become cloudy. This is considered the ideal temperature for detergency. For cleaners that have low foam, optimal detergency is at the cloud point. for foaming cleaners, optimal detergency is at or beneath the cloud point, or just at the point of the cloud point. Phase separation can be prevented by agitation of low foaming cleaners. agitated.
The proportion of nonionic surfactants that are hydrophilic and hydrophobic is the determinant of the temperature of cloud points. Some cloud points are at room temperature and others are extremely high. Certain nonionic surfactants do not have a cloud point because they have a very high ratio of hydrophilic the hydrophobic moiety.
Cationic Surfactants
Cationic surfactants possess a positive charge on their hydrophilic side. The positive charge makes them valuable in anti-static items, such as fabric softeners. They are also used as antimicrobial agents and are frequently included in disinfectants.
Cationic surfactants cannot be used together with anionic surfactants. Combining negatively charged cationic and anionic surfactants with negative charges could result in them losing their effectiveness. Cationic and nonionic surfactants, but, they are compatible.
Amphoteric Surfactants
The amphoteric end of hydrophilic surfactants is charged with an additional charge that is either positive or negative. Dual charges cancel one another, creating the charge known as the zwitterionic. The pH of any solution will determine how the amphoteric surfactants react. The IRO Surfactant in acidic solutions, are positively charged and behave like cationic surfactants. They develop a negative charge in alkaline solutions that is comparable to anionic surfactants.
Amphoteric surfactants are often utilized in personal care products such as shampoos and cosmetics. Some of the most frequently-used amphoteric surfactants include betaines and amino oxides.
